Reactor
Comprehensive virtual reactions to explore the available chemical space at large scale.
Summary
Generate chemically feasible products
Based on defined reaction schemas Reactor performs virtual reactions on a large number of reactants and generates the corresponding products. Detailed reaction scheme definition enables incorporating organic chemistry knowledge to guide the virtual enumeration. The application is available in the form of a Desktop application, Command Line Tool, in workflow tools (KNIME, Pipeline Pilot) and as Java or REST API for programmatic access.

Meet with Us
Download
Features
Reaction synthesis on your desktop
Reactor is a high performance, integrable reaction enumeration engine. It works with generic reaction equations that can be drawn in Marvin or defined and imported in various different formats, including among others: SMIRKS/SMARTS strings, RDF, RXN and MRV files. The Reactor package includes a large and continuously growing library of organic chemical reactions that can be utilized directly, without any further configuration.

Features
Define your chemical reactions
By using generic reaction equations, virtual synthetic compound libraries can be generated under full manual control. Therefore, users have the opportunity to draw and edit reactants directly, and to select chemically meaningful products from the output of the enumeration process - by using their chemical intuition on the fly. This approach is particularly advantageous for enumerating small, focused libraries.
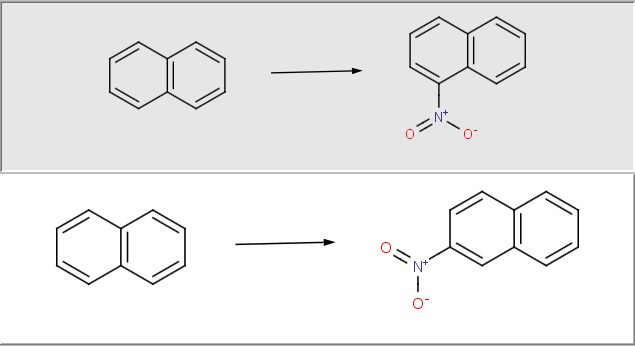
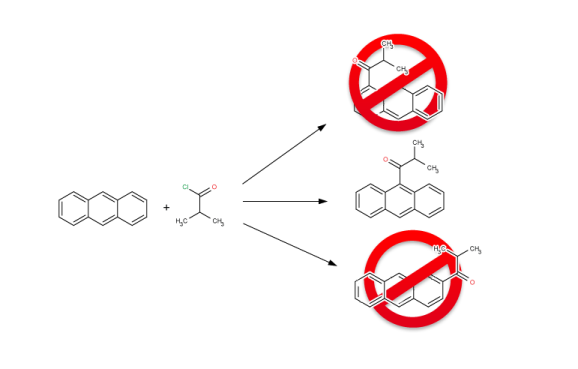
Features
Get synthesizable molecules by proper reaction rules
The reaction rules are defined in Chemical Terms, Chemaxon’s scripting language that is designed to add chemical intelligence to cheminformatics applications. Through Chemical Terms a large number of calculated properties can be included in the reaction rules to produce valid compound libraries. Besides calculating physicochemical properties on-the-fly, the Chemical Terms language also supports importing arbitrary fields from the input reactant files used in the evaluation of the reaction rules.
Features
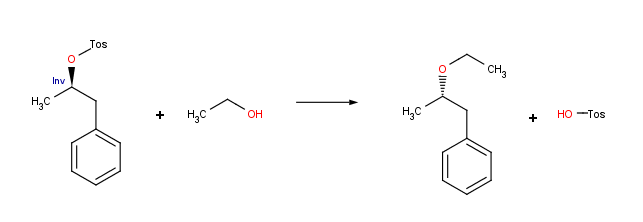
Stereochemically aware reactions
Reaction schemes can include stereochemical information. Reactor is capable of handling both tetrahedral and double bond stereochemistry flexibly. In addition, inversion and retention centers, as well as cis-trans configuration changes can be determined within Reactor’s smart reaction schemes. Prochiral reaction schemes are also supported since version 5.5, allowing the user to manage syn/anti additions.
Features
Library design
Reactor can be set up to carry out simple sequential enumeration, in which case reactants are virtually reacted with each other in the order they are present in the input files. Reactor is also capable of combinatorial enumeration, generating combinatorial virtual synthetic libraries. Users also have the option to exclude unwanted products from the enumeration results manually, restricting the outcome of the reaction enumeration process to the desired main products. Reactor supports the generation of product or reaction libraries in a large variety of different output formats.
Features
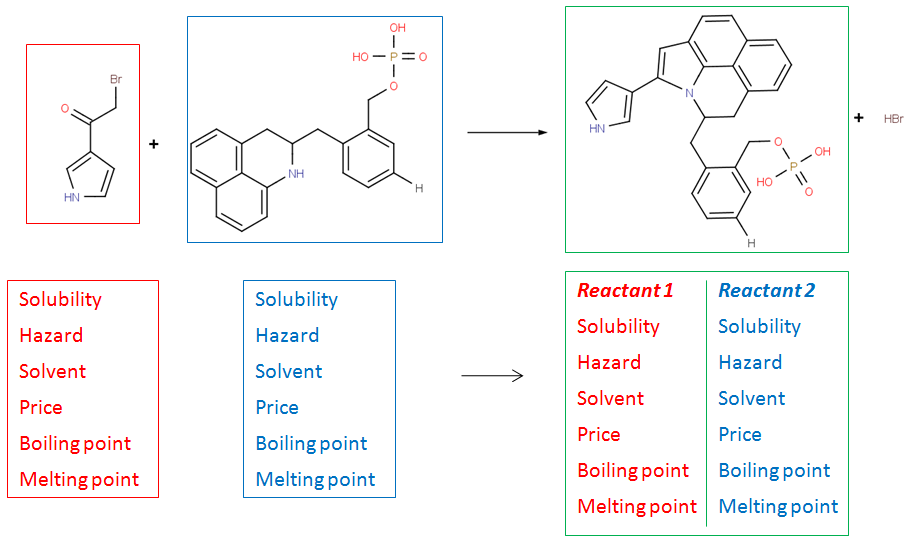
Create rich molecular libraries
Reactor has the option to copy arbitrary property fields from the input reactant files to the results. These can include e.g. solubility or availability information of the reactants. Also, Reactor can generate synthesis codes for each reaction in the enumeration process containing selected information from the reaction scheme and the reactants.
Features
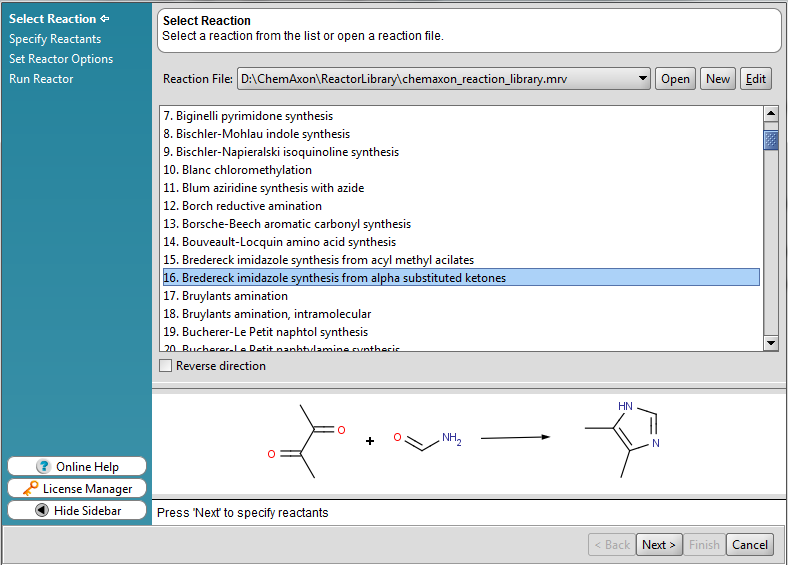
A wizard-like user interface
The standalone version of Reactor has a clean and straightforward graphical user interface for reaction enumeration process configuration. The GUI leads users step by step through the whole configuration process of the virtual chemical reaction.
Features
Accessibility on various platforms
Reactor is available as a standalone application, as well as integrated into Instant JChem, JChem Microservices, JChem for Office, and workflow management tools KNIME and Pipeline Pilot. In its standalone version, it can be used as a GUI application, as a command line application, and also as a full-featured Java API. Reactor is platform-independent, it is available on Windows, Mac OS and Linux systems.
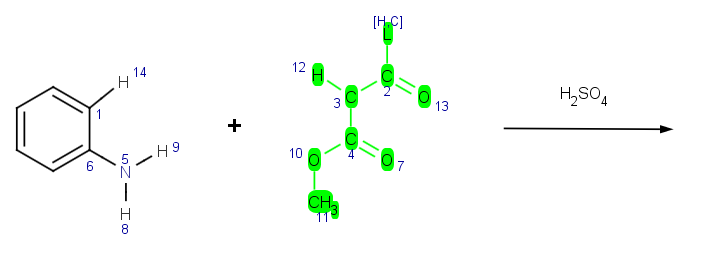
Features
Create and test your reactions
Reactor has an integrated reaction sketcher and editor tool. Users can create their own reaction schemes and add corresponding reaction rules by using the Chemical Terms language. The created reaction schemes can be easily tested and the reaction rules can be validated using the integrated reaction testing tool in Reactor.
Knowledge Hub
Resources
Learn more about Reactor.
Related Products
Marvin
Full featured chemical editor for all platforms
Chemical Naming
Convert chemical names into structures
Markush Technology
Smart assistant for patent claim drafting and Markush analysis
Chemical Structure Representation
Standardization and correction of chemical structures
Chemicalize
Calculate properties instantly, search chemical data, and draw molecules online
Compound Registration
Normalize, check, validate and register chemical compounds
Reactor
High performance virtual synthesis engine
JChem for Office
Chemical structure handling, data analysis, visualization and reporting capabilities within MS Office
Design Hub
A single platform that connects scientific rationale, compound design and computational resources
JChem Engines
Search through tens of millions of chemical compounds and receive relevant query hits in seconds.
Calculators and Predictors
Execute high quality physico-chemical calculations and predictions.
Compliance Checker
Identify controlled substances with Compliance Checker and assign HS tariff codes with cHemTS - the easy way to comply with chemical regulations.
Discovery Tools
From clustering and diversity analysis for chemical libraries to 2D and 3D molecular screening
Instant JChem
Create, explore and share chemical data
ChemCurator
Computer-assisted chemical information extraction and analysis